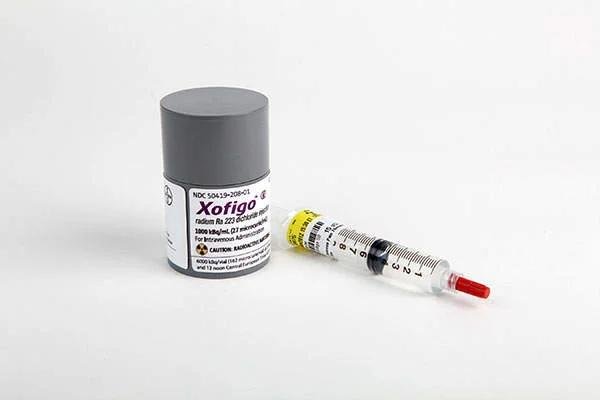
What is Xofigo?
Xofigo (radium 223 dichloride) is an intravenous injection for treating prostate cancer. in cases where surgical or hormonal treatment that reduces testosterone is no longer effective and the cancer has spread into bones but is not spreading to other organs in the body. Xofigo contains a radioactive component called radium 233, which is found in the bone area of cancer and kills cancerous cells.
Xofigo is a drug that acts like calcium, and it is transported to bones that are growing rapidly in the area where bone cancer has spread to the body. When Xofigo is bonded to an area of bone cancer, it releases radiation that damages the DNA of cancerous cells. This destroys prostate cancer cells.Its half-life, Xofigo, has been set at 11.4 days.
Warnings
Bone Marrow Suppression: Blood counts should be evaluated prior to the beginning of treatment and before each dosage of Xofigo. Refrain from taking Xofigo when hematologic levels are not restored within 6–8 weeks following treatment. Be sure to monitor patients who have damaged bone marrow reserves closely. The medicine should be stopped when patients experience life-threatening complications despite supportive measures.
Before you take this drug
The male fertility of men and pregnancies
The safety and effectiveness of Xofigo are not known for females. The drug can harm a foetus when given to a pregnant woman. It is crucial to inform women and pregnant females about the reproductive risk and the potential danger to the foetus. Pregnancy is not recommended.
Male patients should be careful not to get female partners pregnant while receiving treatments with Xofigo and for 6 months following stopping treatment. Patients who are male should use condoms, and their female companions (of potential reproductive capacity) should be using an effective contraceptive method during and for six months after the end of treatments with Xofigo.
Based on the way Xofigo operates, it could hinder the ability of males to father children if they have reproductive potential.
Bone Marrow Suppression:
It is essential to stay in compliance with the blood cell count monitoring schedule during treatment with Xofigo. If you notice signs of bleeding or infection, contact your physician.
Increased Fractures and Mortality in Combination with Abiraterone plus Prednisone or Prednisolone:
Patients using Xofigo have an increase in bone fractures and mortality when used in combination with abiraterone acetate and prednisone or prednisolone. It is recommended to talk with your doctor about any other medications you are currently taking to treat prostate cancer.
Fluid Status:
It is vital to stay hydrated and be aware of your intake, fluid levels, and output of urine while receiving treatment with Xofigo. It is important to report any signs of hypovolemia, dehydration, urinary retention, or renal failure or insufficiency to the doctor.
Personal contact with radiation and exposure to other people
There are no limitations on the personal interaction (visual as well as physical) with people who have received the injection following the injection of Xofigo. You must adhere to good hygiene guidelines during the time you receive Xofigo as well as for at least one week after your last injection to reduce the radiation exposure of body fluids and other bodily fluids for household members as well as caregiveWhen you are using a toilet, it needs to be flushed multiple times after every use. Clothing that is soiled by urine or faecal matter must be cleaned promptly and separated from other clothes. The carers should follow universal precautions for the care of patients, including barriers and gloves while handling bodily fluids, in order to prevent contamination. When dealing with bodily fluids, wearing gloves as well as hand washing can protect carers.
How to take Xofigo?
The procedure involves giving Xofigo in a one-minute injection into a vein every four weeks, for six total injections. The treatment will be carried out in a health facility.
After treatment, you're able to depart the hospital and resume your regular routine. There aren't any restrictions on contacts (visual and physical) with others. This means that you are allowed to hug your loved ones and relatives.The dose of Xofigo has been set at 50 kBq (1.35 microcurie) per kg of body weight.
Dosage Forms and Strengths
Single-use vial having a dose of 1000 1 kBq/mL (27 microcurie/mL) as of the date of reference with an overall radioactivity of 6,600 KBq/vial (162 microcurie/vial) at the time of reference.
Side effects of Xofigo
One of the most serious side effects of Xofigo is the suppression of bone marrow. It is essential to conduct your blood tests regularly. It is important to report any signs of bleeding or infection to your doctor.
The most frequently reported adverse effects that afflicted 10 percent or more of those who received Xofigo were vomiting, nausea, diarrhoea, vomiting, and peripheral oedema.The most frequent abnormalities in the laboratory of haematology (> 10 percent) were lymphocytopenia, anaemia, leukopenia, thrombocytopenia, and neutropenia.
To report suspected adverse reactions, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Interaction with other drugs
There have been no formal studies of interactions between drugs in clinical trials.Subgroup analyses showed that the use of bisphosphonates or calcium channel blockers in the same manner as Xofigo did not impact the safety or effectiveness that were demonstrated by Xofigo in the clinical study.